| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-07-13 19:50:51 UTC |
|---|
| Update Date | 2016-07-19 23:06:35 UTC |
|---|
| Lmdb | LMDB00483 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
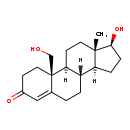
| Common Name | 19-Hydroxytestosterone |
|---|
| Description | 19-Hydroxytestosterone is an intermediate in Androgen and estrogen metabolism. 19-Hydroxytestosterone is the 4th to last step in the synthesis of 16-Glucuronide-estriol. It is generated from Testosterone via the enzyme cytochrome P450 (EC 1.14.14.1) and then converted to 19-Oxotestosterone. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 17beta,19-Dihydroxyandrost-4-en-3-one | HMDB |
|
|---|
| Chemical Formula | C19H28O3 |
|---|
| Average Molecular Weight | 304.4238 |
|---|
| Monoisotopic Molecular Weight | 304.203844762 |
|---|
| IUPAC Name | (2S,10R,14S,15S)-14-hydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | (2S,10R,14S,15S)-14-hydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| CAS Registry Number | 2126-37-6 |
|---|
| SMILES | [H]C12CC[C@H](O)[C@@]1(C)CCC1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12CO |
|---|
| InChI Identifier | InChI=1S/C19H28O3/c1-18-8-7-16-14(15(18)4-5-17(18)22)3-2-12-10-13(21)6-9-19(12,16)11-20/h10,14-17,20,22H,2-9,11H2,1H3/t14-,15?,16?,17-,18-,19+/m0/s1 |
|---|
| InChI Key | YLTCTXBDDHSLCS-HRCCTRRKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 19-hydroxysteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Cyclic alcohol
- Cyclic ketone
- Secondary alcohol
- Ketone
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00g0-0190000000-6e4fc7b300ad16c455cd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-1203900000-4f90d6dd0388b3a16017 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0093000000-e6de474519f1a50b8a29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0670-0090000000-e998b159f6f17e76d6ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0550-3590000000-c2362be82382a74f0ccb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0059000000-22c9035dbc2909953680 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zmr-0094000000-a86f4275aef3909e642b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fs-0090000000-a227eb144908c8f752ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0019000000-96c876787125565560bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0892000000-64923385891f1c2ddf28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0900000000-9b70a6d7d0da7c6a6b3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0019000000-28d038f60bc762557a4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0039000000-b8ab70909d16db966311 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-0090000000-21047d70207f0666ac31 | Spectrum |
|
|---|