| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-08-06 05:39:25 UTC |
|---|
| Update Date | 2020-03-27 15:55:06 UTC |
|---|
| Lmdb | LMDB01010 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | 5Z,8E-3-hydroxytetradecadienoylcarnitine |
|---|
| Description | (5Z,8Z)-3-Hydroxytetradecadienoylcarnitine belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. Thus, (5Z,8Z)-3-hydroxytetradecadienoylcarnitine is considered to be a fatty ester. Based on a literature review a significant number of articles have been published on (5Z,8Z)-3-Hydroxytetradecadienoylcarnitine. |
|---|
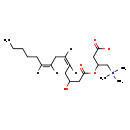
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-{[(5Z,8Z)-3-hydroxytetradeca-5,8-dienoyl]oxy}-4-(trimethylammonio)butanoate | ChEBI | | 5-cis,8-cis-3-Hydroxytetradecadienoylcarnitine | ChEBI | | 3-{[(5Z,8Z)-3-hydroxytetradeca-5,8-dienoyl]oxy}-4-(trimethylammonio)butanoic acid | Generator |
|
|---|
| Chemical Formula | C21H37NO5 |
|---|
| Average Molecular Weight | 383.529 |
|---|
| Monoisotopic Molecular Weight | 383.267173295 |
|---|
| IUPAC Name | 3-{[(5Z,8Z)-3-hydroxytetradeca-5,8-dienoyl]oxy}-4-(trimethylazaniumyl)butanoate |
|---|
| Traditional Name | 3-{[(5Z,8Z)-3-hydroxytetradeca-5,8-dienoyl]oxy}-4-(trimethylammonio)butanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]\C(CCCCC)=C(/[H])CC([H])=C([H])CC(O)CC(=O)OC(CC([O-])=O)C[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C21H37NO5/c1-5-6-7-8-9-10-11-12-13-14-18(23)15-21(26)27-19(16-20(24)25)17-22(2,3)4/h9-10,12-13,18-19,23H,5-8,11,14-17H2,1-4H3/b10-9-,13-12- |
|---|
| InChI Key | LVNITLZCNUEXQK-UTJQPWESSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Acyl carnitines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyl-carnitine
- Beta-hydroxy acid
- Dicarboxylic acid or derivatives
- Hydroxy acid
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid salt
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic zwitterion
- Organic salt
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Amine
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-25c2db346e4877a52c82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-9005000000-6ffa7ee4e5ee878cc6e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-e9262cbaff8cb4ad0ba6 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|